September 10, 2024 7 min to read
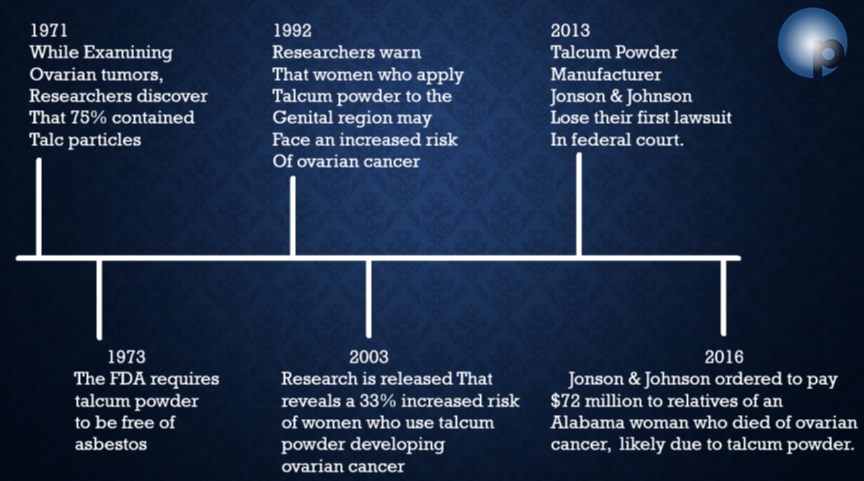
Johnson & Johnson’s Long History of Lawsuits
Category : DISCOVERY, SCIENCE
The European Union’s (EU) drug regulator, the European Medicines Agency (EMA), has discovered a potential link between Johnson & Johnson’s Janssen COVID-19 vaccine and incidents of blood clots in an individual’s deep veins. The issue was highlighted in a Pharmacovigilance Risk Assessment Committee (PRAC) meeting.
1. Johnson & Johnson’s Vaccine Linked to Blood Clotting Condition
https://www.oppenheimlaw.com/johnson-and-johnson-talcum-powder-lawsuit/
https://www.nbcnews.com/health/health-news/johnson-johnson-vaccine-linked-28-cases-blood-clots-cdc-reports-n1267128
https://www.kgw.com/article/news/health/coronavirus/woman-reports-multiple-blood-clots-after-johnson-johnson-shot/283-7fb4ef05-ea38-486f-a3db-4b7ffe5780e8
https://www.fox17online.com/news/local-news/ionia/ionia-co-womans-death-after-getting-j-j-vaccine-reported-to-the-cdc
https://www.law.com/2020/06/23/appeals-court-lowers-4-7b-talc-verdict-to-2-1b-but-cites-jjs-evil-motive/?slreturn=20210509124429
https://www.theguardian.com/business/2020/may/19/johnson-johnson-baby-powder-us-canada
https://www.forbes.com/sites/korihale/2020/10/14/johnson–johnsons-100-million-baby-powder-lawsuit-settlement-is-overdue-for-black–hispanic-women/